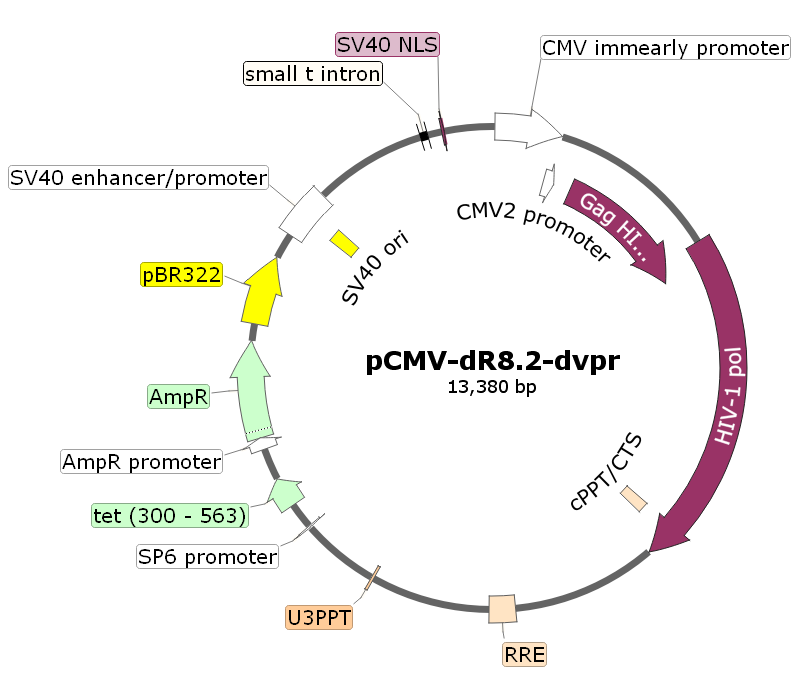
pCMV-dR8.2-dvpr
pCMV-dR8.2-dvpr
编号 | 载体名称 |
北京华越洋VECT231141 | pCMV-dR8.2-dvpr |
pCMV-dR8.2-dvpr载体基本信息:
载体名称: | pCMV-dR8.2-dvpr 其它名称 pCMVdR8.2 dvpr、 pHR' CMV 8.2 deltaR |
质粒类型: | 哺乳动物载体;第二代慢病毒包装;双质粒包装系统 |
高拷贝/低拷贝: | 高拷贝 |
克隆方法: | 限制性内切酶,多克隆位点 |
启动子: | CMV |
载体大小: | 13380 bp |
5' 测序引物及序列: | CMV fwd 5’CGCAAATGGGCGGTAGGCGTG 3’ |
3' 测序引物及序列: | -- |
载体标签: | 无 |
载体抗性: | Ampicillin |
筛选标记: | 无 |
克隆菌株: | DH5α,HB101等 |
宿主细胞(系): | 包装细胞系如293T |
备注 | 第二代慢病毒包装载体pCMV-dR8.2-dvpr需要与信封载体pCMV-VSVG 、pCMV-VSV-G 共同使用,使用方法见下文。用来包装第二代、第三代慢病毒表达载体。 |
稳定性: | 瞬表达 |
组成型/诱导型: | 组成型 |
病毒/非病毒: | 非病毒 |
pCMV-dR8.2-dvpr载体质粒图谱和多克隆位点信息:

pCMV-dR8.2-dvpr载体简介:
双质粒系统慢病毒包装质粒pCMV-dR8.2-dvpr使用方法
Day 1
1. Plate 5 x 105 293T cells in 6 cm2 dishes containing 5 mL of media. (This can be scaled up if desired)
Day 2
1. Set up (use polypropylene tubes for this; polystyrene tubes DO NOT work!):
1 µg retroviral DNA encoding gene X
1 µg packaging plasmid Mix
the packaging plasmid (pCMV-dR8.2-dvpr) at a 8:1 ratio with the envelope plasmid (pCVM-VSV-G)-a total of 1µg
DME without serum to 94μL total
6 μL Fugene
Mix and wait 15 to 30 minutes at room temperature
Add to 293T cells without touching the sides of the dish (DO NOT CHANGE MEDIA)
If you are using amphotropic virus then move immediately to BL2+ in a secondary container, which has an absorbent material.
(This does not mean a couple of hours; it means Immediately!). The rest of this protocol is the same for all viruses---the BL2+ safety practices are in place if you are using amphotropic viruses
Day 3
1. Change the media to whatever media you wish to use when infecting target cells. 293T cells are easily detached so remember not to put the media directly onto to cells, but rather “run” it down the side of the dish. Remember that you will get the highest titer virus when your cells are “happy.”
2. Plate out your target cells
Day 4
1. Remove the medium from the 293T cells and use a 0.45 u syringe filter to remove any 293T cells. DO NOT use the 0.2 u filter, as it is likely to shear the envelope from your virus
making it noninfectious
Note: After filtering, the filter should be removed and placed in the biohazard bag in the hood and the syringe rinsed with bleach and decontaminated for a minimum of 20 min. It is useful to place a plastic beaker with bleach in the hood in advance
2. Add 8 to 10 μg/ml of polybrene (Hexadimethrine bromide) or protamine sulfate to the target cells
3. Carry out infection for 1 to 4 hours. Remove virus and replace with fresh media
Note: If you wish to do a second infection the following day, it is important to put fresh media on the cells and not let the virus remain on the cells overnight. The media contains huge amounts of envelope, both associated and unassociated with viral particles, which will bind all the cell surface receptors required for virus adsorption, resulting in their down-regulation. Hence, if you don’t change the media after the initial infection, very few receptors will be available for the next round of infection. In addition, very few cells tolerate the presence of high levels of the VSV-G envelope for extended periods of time (i.e. a lot of your cells may die)
Day 5 +
1. Allow the cells to recover and begin to express the virus-encoded genes. The cells usually require 48 hours for this to occur
2. Add drug if you are scoring for the presence of a vector that carries a drug resistance marker. Prior to this step it is advisable that you titrate the drug to be used for selection in order to know precisely how much to add. In addition, it is necessary to bring an extra plate of uninfected cells (often referred to as “canaries”) which will function as a positive control in the kill assay. Add drug to both plates. When your canaries are dead, you can remove the drug.
pCMV-dR8.2-dvpr载体序列
pCMV-dR8.2-dvpr其他相关慢病毒载体: